How we test our hypotheses
To define how neural circuits for food consumption and behavioral motivation in the mammalian brain are merged to regulate food intake, Zhang lab uses a variety of techniques including optogenetics, chemogenetics, electrophysiology, immunocytochemistry, and fiber photometry in our experimental researches.
Optogenetics
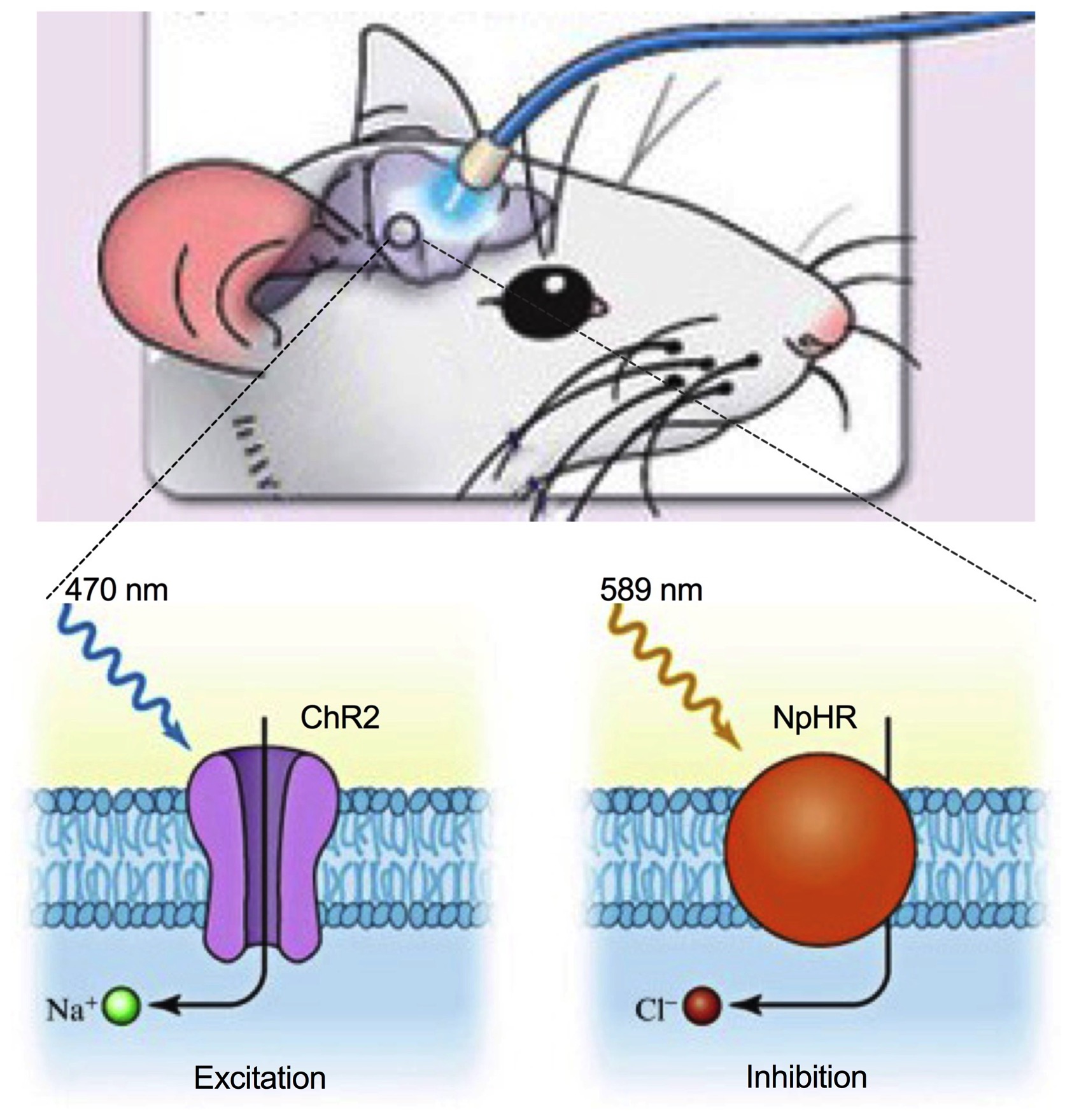
Optogenetics is a biological technique that involves the use of light to control neurons that have been genetically modified to express light-sensitive ion channels. This technology allows us to manipulate neuronal activity in specific cells and circuits for behavioral and functional observation in a fast and reversal way. To do this, we first inject AAV-based viral vectors to specific brain regions for the Cre-dependent expression of ChR2 (excitatory) or NpHR in transgenic Cre mice. One month after virus injection, fiber optics are implanted to target ChR2- or NpHR-expressing neurons or their synaptic terminals for photostimulation during behavioral tests. The most important advantage of optogenetics is that we can selectively manipulate one of the projections by the same neurons to test circuit-dependent behavioral control. We use this technology in combination with slice electrophysiology to dissect functional neural circuits in controlling food intake and feeding-related behaviors.

Chemogenetics
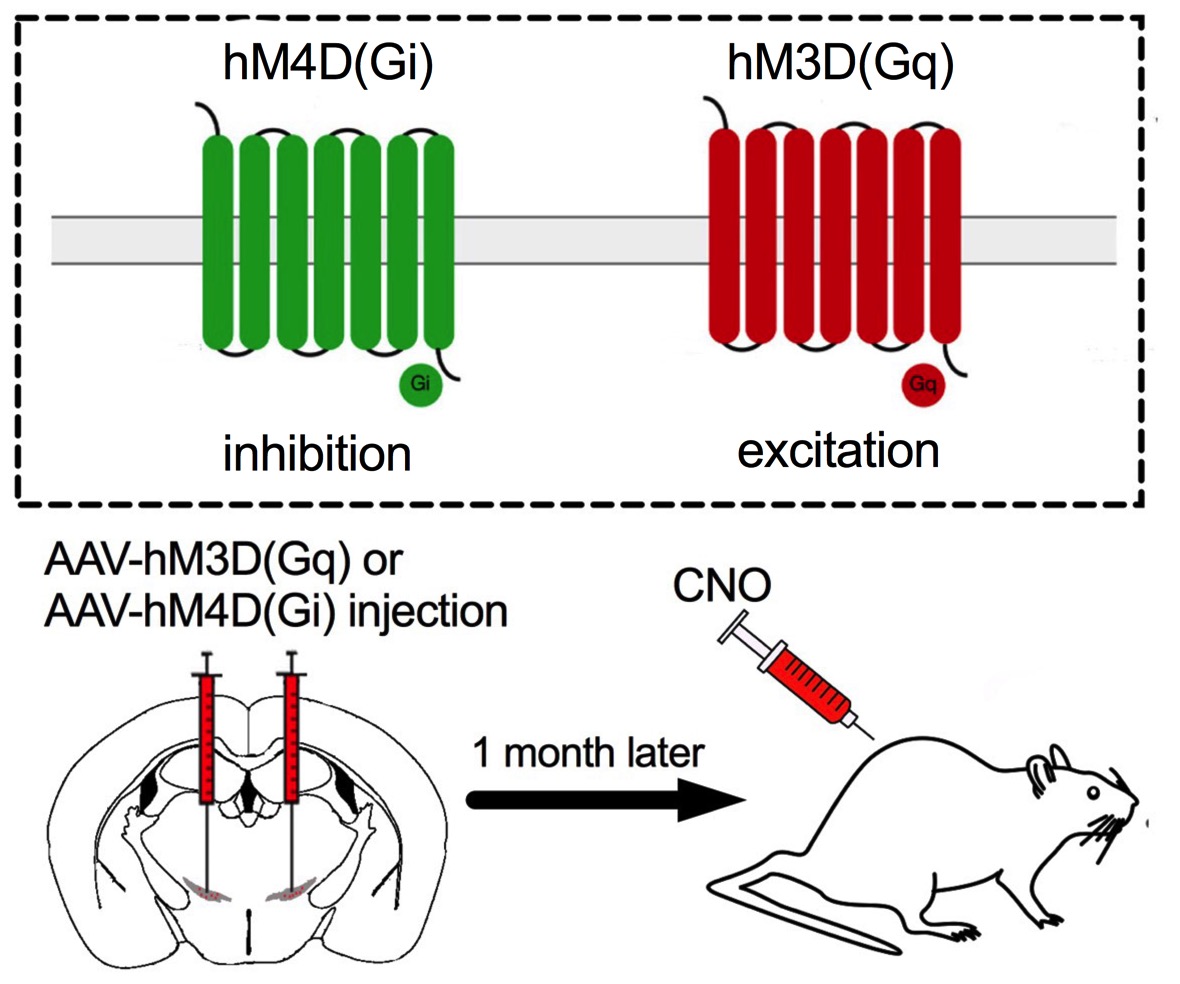
Chemogenetic technology uses Designer Receptors Exclusively Activated by Designer Drugs (DREADDS), which are G-protein-coupled receptors that are genetically modified in such a way that they are no longer responsive to the endogenous ligand, but instead are activated by designer drugs (clozapine N-oxide, CNO). This technology allows us to manipulate neuronal activity for a long-time effect following intraperitoneal injection of CNO in a non-invasive way. To employ this technology, we first inject AAV vectors to induce cre-dependent expression of hM3D(Gq) or hM4D(Gi) in targeted neurons. One month after virus injection, mice are ready for behavioral experiments with an intraperitoneal injection of CNO. Our lab uses this technology to reveal the novel role of central neurons in the regulation of food intake and feeding motivation.

Fiber photometry
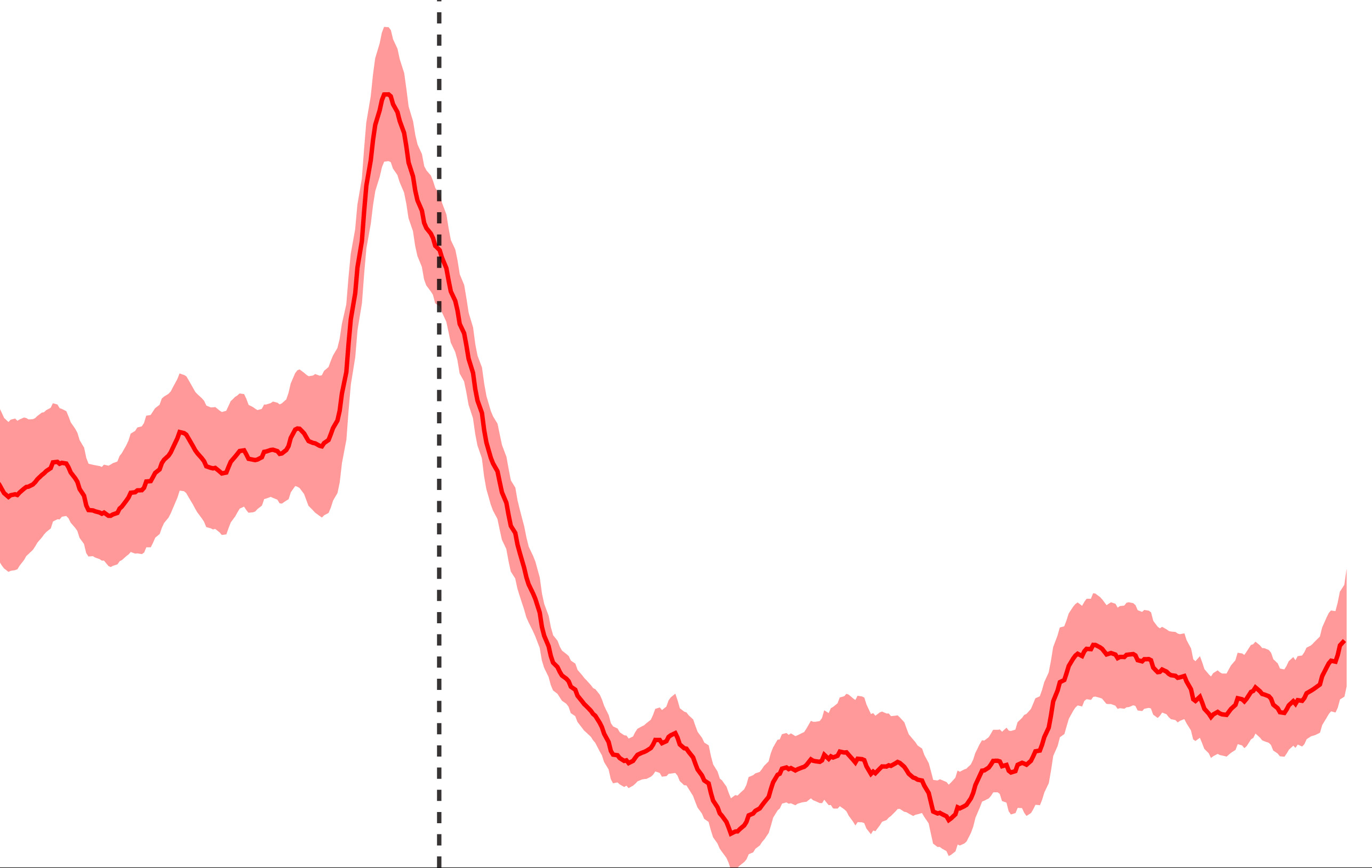
Fiber photometry is an optical technique that enables scientists to measure chemical signaling (such as intracellular calcium level using calcium indicator GCaMP and neurotransmitter level using neurotransmitter sensors) in the brain of a freely-moving animal. Due to the high sensitivity of GCaMP to the fast change of calcium levels, this technology allows us to track the real-time neuronal activity in behaving animals so that we can detect the relationship of specific neurons and behaviors such as feeding. Our lab uses fiber photometry to understand how feeding-related neurons regulate food intake (for example feeding motivation vs. food consumption).

Slice electrophysiology
The patch-clamp technique is a laboratory technique in electrophysiology used to study neural activity by measuring membrane potential or currents. We use this tool in our lab to study how feeding-related neurons and synaptic transmission are regulated by neurotransmitters, neuropeptides, metabolic hormones, and physiological states of energy. In addition, we combine slice electrophysiological recording with optogenetic and chemogenetic manipulations to dissect functional neural circuits for feeding regulation.

Immunocytochemistry
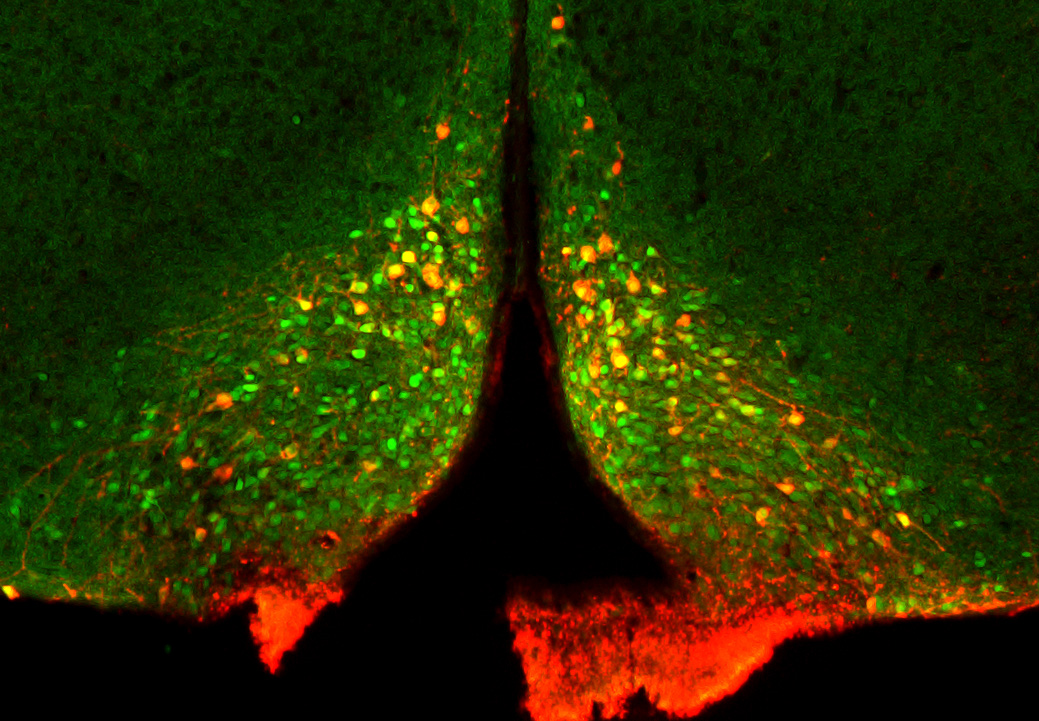
Immunocytochemistry is a traditional method that is used for the anatomical identification of neuronal types, protein expression, and neural connections. Our lab uses immunocytochemistry to identify protein expression in feeding-related neurons of both wide-type and transgenic mice so that we can understand the mechanism by which these neurons regulate food intake. In addition, we combine immunocytochemistry with neural circuit tracers (such as Cholera toxin subunit B and retrograde viral vectors) to identify functional feeding circuits.

Behavior
Our work mainly focuses on feeding-related behaviors. We not only measure food intake but also test feeding motivation. We developed feeding experimental devices (FEDs) to measure meal patterns including meal size and meal frequency. We use operant chambers to test the motivation of mice for food rewards. We also observe the emotion and motivation of mice such as anxiety and locomotion in specific conditions since both emotion and motivation have been revealed to affect food intake. We employ optogenetic and chemogenetic manipulations to intervene in specific feeding-related behaviors and fiber photometry to track neuronal responses to real-time behavioral change.


